Overview
ASD141 is a novel, innate immune checkpoint inhibitor that commands the innate immune system to coordinate a renewed attack on solid tumors.
ASD141 works by:

Reinvigorating the cells that coordinate an immune response in the tumor microenvironment.

Improving the interface between innate and adaptive immunity.

Driving active antigen-presenting cells to the tumor environment.
Approximately 75% of patients do not respond to current immune checkpoint inhibitors (ICIs)1,2. By commanding a coordinated, innate immune response, ASD141 accesses a novel therapeutic route that could reach some of these patients.
Therapeutic Area
Immuno-oncology (IO)
Development Stage
Phase I clinical trial
ClinicalTrials.gov ID: NCT06235437
Seeking
Investment
Licensing
Co-development
The Challenge
As solid tumors grow, they can adopt mechanisms to suppress the immune system. One method is to induce the release of TLT-1 from platelets.7 TLT-1 binds to CD11b receptors on immature, innate myeloid cells in the tumor microenvironment and switches them to an immune suppressive state.3 By the time a tumor is detectable, the tumor microenvironment often contains high levels of immune suppressive cells and immune exhaustion has already set in.
Although ICIs have shown great success in treating some cancers by elevating an adaptive immune response through reactivated T cells, they only work for around 30% of cancer patients. Effective ICI therapy is not without its problems too, cytokine release syndrome (CRS)4 has been reported in around 4.6% of patients receiving ICIs5, where elevated levels of cytokines lead to immune hyperactivation with life-threatening consequences. Anti-CTLA4 therapy has also been linked to autoimmune disease through the depletion of regulatory T cells6.
The main challenge for ICI therapy is that the drug acts directly on the adaptive immune system bypassing the mediation by the innate immune system. When drugs are used to directly amplify the numbers and activation of T cells, unchecked, overactivation can occur.
The Solution
Our lead asset ASD141, a first-in-class monoclonal antibody, blocks the innate immune checkpoint TLT-1 signaling and modulates tumor microenvironment (TME). TLT-1 is a platelet-derived protein highly expressed in a diverse spectrum of tumors, especially in tumors known to be resistant to current checkpoint inhibitors (e.g. anti-PD1). In the pre-clinical studies, ASD141 showed anti-tumor activities in humanized mouse models when used alone or in combination with anti-PD1 or anti-CTLA-4 antibodies. This asset is currently at Phase I dose escalation trial.
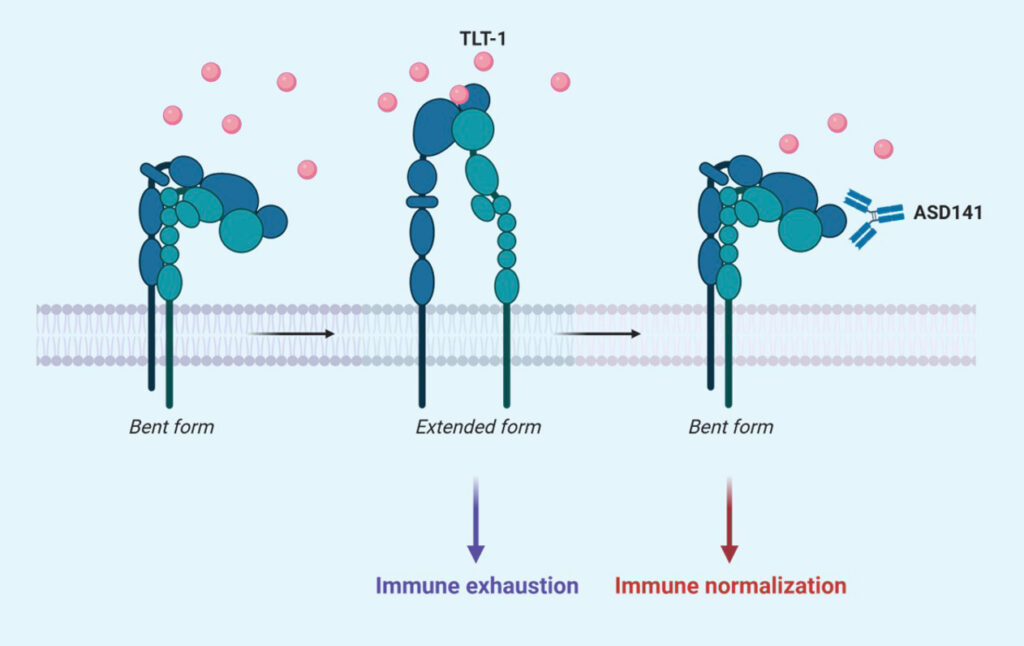
Mode of Action (MoA)
The mechanism of action of ASD141 monoclonal antibody is directly through blocking the binding of TLT-1, an immune suppressive protein, to its receptor on myeloid cells, and thus promotes immune responses toward tumors.
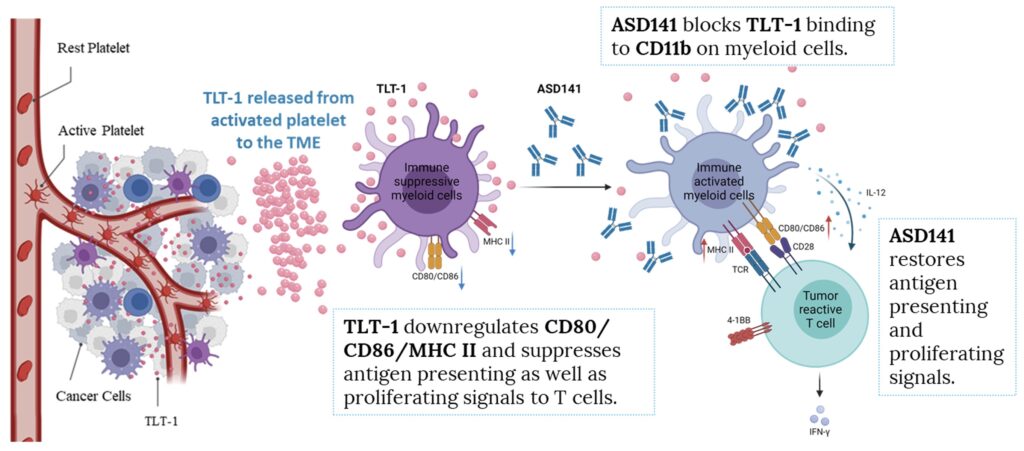
Details
Our animal pharmacological studies demonstrated that ASD141 reversed the immune-suppressive microenvironment in mouse colon cancer model and inhibited tumor growth when used alone or combined treatment with T cell-targeting checkpoint inhibitors (e.g., anti-PD1 or anti-CTLA-4 antibodies).
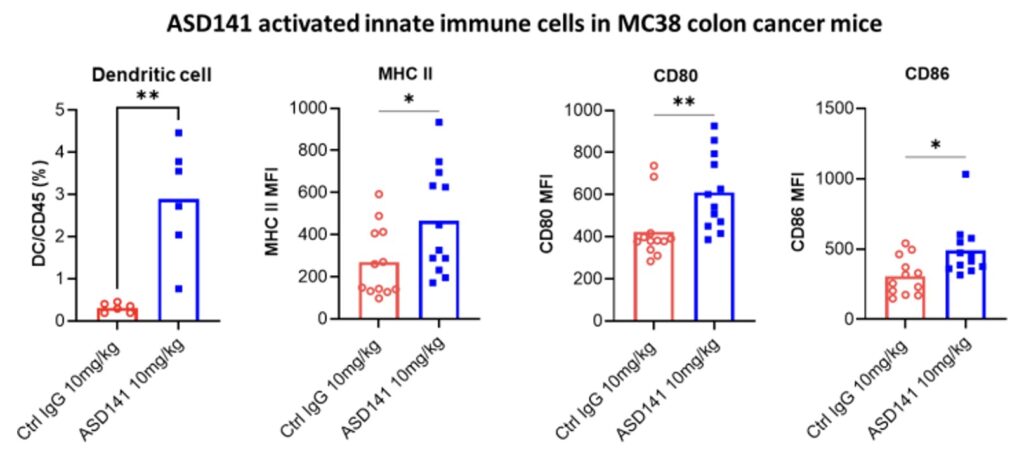
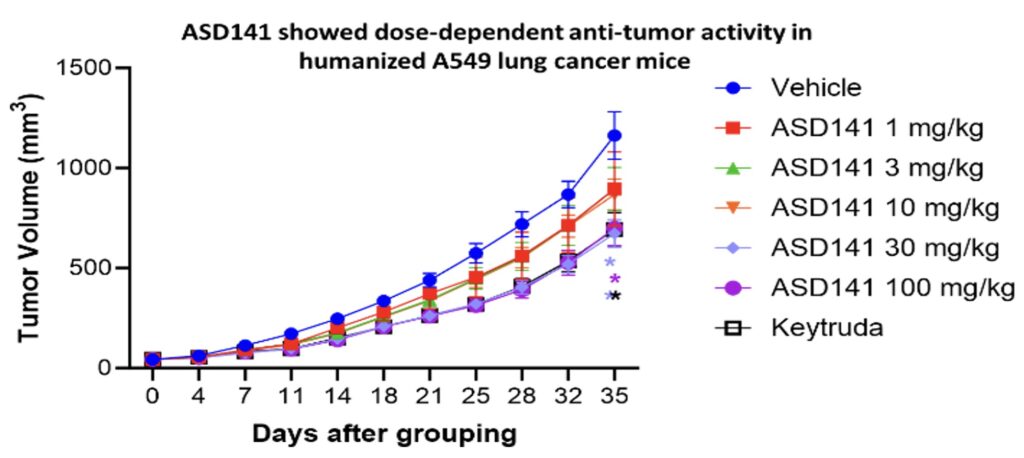
In the humanized mouse model, ASD141 monotherapy resulted in a dose-dependent inhibition on the growth of human A549 lung tumor. ASD141 is therefore expected to benefit patients with various types of solid tumors. The US FDA approved the Phase I dose-escalation study IND, and patient enrollment began in December 2024. The study demonstrates safety and tolerability, with preliminary efficacy data expected in Q4 2025.
References
- Schoenfeld, Adam J., and Matthew D. Hellmann. “Acquired resistance to immune checkpoint inhibitors.” Cancer cell 37.4 (2020): 443-455. https://doi.org/10.1016/j.ccell.2020.03.017
- Haslam A, Prasad V. Estimation of the Percentage of US Patients With Cancer Who Are Eligible for and Respond to Checkpoint Inhibitor Immunotherapy Drugs. JAMA Netw Open. 2019;2(5):e192535. doi:10.1001/jamanetworkopen.2019.2535
- Ascendo unpublished data
- Shimabukuro-Vornhagen, Alexander et al. Cytokine release syndrome. Journal for immunotherapy of cancer vol. 6,1 56. 15 Jun. 2018. doi:10.1186/s40425-018-0343-9
- Tay Sen Hee et al. Cytokine Release Syndrome in Cancer Patients Receiving Immune Checkpoint Inhibitors: A Case Series of 25 Patients and Review of the Literature. Frontiers in Immunology. 2022. 13. doi: 10.3389/fimmu.2022.807050
- Bertrand A, Kostine M, Barnetche T, Truchetet ME, Schaeverbeke T. Immune related adverse events associated with anti-CTLA-4 antibodies: systematic review and meta-analysis. BMC Med. 2015 Sep 4;13:211. doi: 10.1186/s12916-015-0455-8
- , . The theory of tumor ecosystem. Cancer Commun. 2022; 1– 22. https://doi.org/10.1002/cac2.12316
